arthramid
Arthramid VetArthramid(r)Vetis is a patented and unique hydrogel that provides an innovative and safe treatment for veterinarians. OverviewThe latest generation of joint treatment2.5% cross-linked polyacrylamide hydrogel82.5% of patients successfully resolving joint lameness that lasts for 24 monthsIntegrates into joint tissue to provide shock absorption for the joint. Non-toxic, biocompatible, safe and non-toxic to the brain. Share Enquire now Product description Arthramid(r)Vetis is used to treat the non-infectious causes of joint pain in animals, which includes both the early and the late stage of osteoarthritis (OA). arthramid
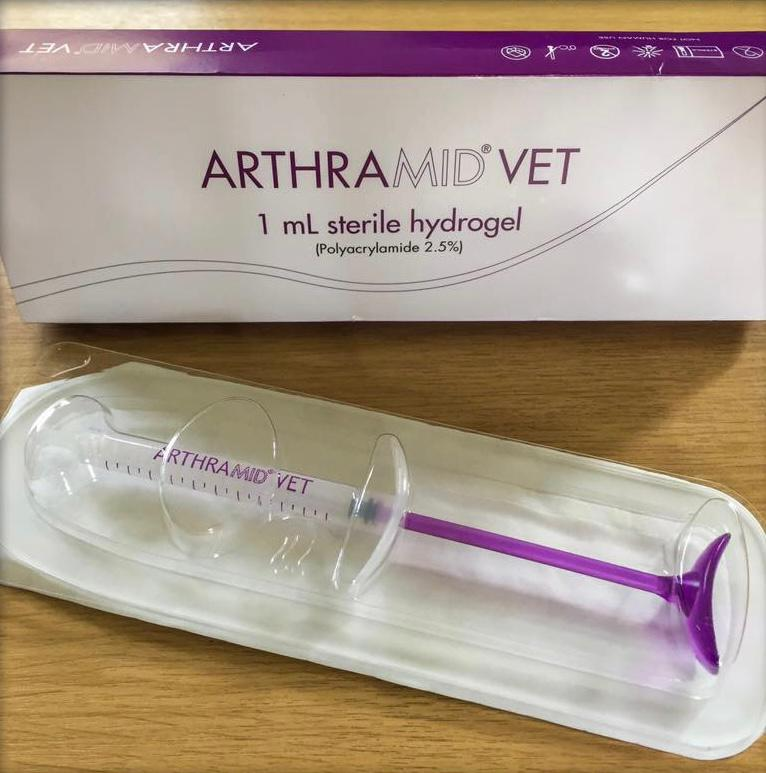
Arthramid(r)Vetis an biocompatibility, absorbable and non-pyrogenic neuro-innocuous, injectable polyacrylamide hydrogel to be used for intra-articular injections of animals. Arthramid(r)Vetconsists of approximately 2.5% cross- linked polyacrylamide and 97.5% water forinjection. The hydrogel comes in a sterilized pre-filled 1 milliliter syringe that is sealed with an Luer Lock fitting.Product in detailAdministrationArthramid(r)Vetis is intended for intra-articular use only.
It is administered by a certified vet who is familiar with the procedure within the intended species. Animals may be sedated in order to reduce anxiety and stress. A strict aseptic protocol must be observed at all times.A 16-23G needle is inserted within the articular canal. The presence of synovial fluid is to be watched. It is important to avoid causing unnecessary damage to the intra-articular tissues, since this could result in an extensive swelling that lasts for 24 or 48 hours.
Remove the protective tip cap from the Arthramid(r)Vetsyringe. Connect the syringe securely into the Luer lock socket on the needle. Be sure that the syringe has been properly placed. If more than 1 mL of Arthramid(r)Vetis required forthe joint, detach the empty Arthramid(r)Vetsyringe from the hypodermic needle and repeat the process until the required dose is delivered.Dosages – EquineDue to Arthramid(r)
Vet’sunique mode of action the following dosage recommendations have been made based on observed clinical responses to treatment;Distal Interphalangeal (DIP/ Coffin)- 1-2 mLsMetacarpo/tarso-phalangeal (Fetlock)- 2 mLsShoulder- 3 mLsStifles- 1-2 mL per compartment or 3-4mLs formedial-femorotibial jointThe results of clinical trials published by the American Heart Association suggest that horses who are not fully responsive to initial treatment could receive another dose 6 to 4 weeks after.Safety studies have shown that the simultaneous treatment of multiple joints within one animal can be secure.
arthramid equine
Module of action – Not all hydrogels of polyacrylamide work exactly the sameThe hydrogels made of polyacrylamide, though typically viewed as being equal, exhibit distinct differences in their the composition, quality of manufacturing and techniques for injection, in addition to their ability to interact with the surrounding tissues. These aspects affect the effectiveness and safety of every hydrogel.When injected into joints, Arthramid(r)Vet,a unique cross-linked 2.5 polyacrylamide hydrogel is able to adhere in the lining of synovial joints by its capability for exchange of water molecules. Over the course of 14 days, the gel is incorporated in the synovial membrane as well as the surrounding tissue of the capsule’s interior by a combination of cells movement and vessel growth, creating the thick, cushion-like skin composed of a vessel-integrated
gel that is covered by a brand new hypercellular synovial cell lining.In the end, Arthramid(r)Vethas an enhancement effect on the synovium and the joint capsule. It improves the elasticity and tensile strength of the joint capsule which increases its ability to carry the load. The theory is that the improvement and cushioning results in a decrease in mechanoreceptor stimulation within the capsule. This also leads to the development of a hypercellular and new synovial cell lining that improves the synovial fluid’s health inside the joint. These actions can help decrease synovitis as well as its subsequent adverse effects.Unlike other hydrogels Arthramid(r)Vetbecomes fully integrated into the surrounding tissue (rather than encapsulated) and is therefore long lasting.
Arthramid(r)Vetdoes not trigger an allergic reaction to foreign bodies or harbor an infections. Arthramid(r)Vetis is not pyrogenic, non-toxic to the brain and is free of heavy metals.Case selectionUnderstanding the complex process that causes joint pain remains a perpetual issue in the clinical setting and like any other disease process it is crucial to make a precise diagnosis. vital.Arthritis is a term used to describe joint inflammation and may develop after one or repeated traumas. The term encompasses synovitis capsuleitis, and osteoarthritis (OA).
These conditions can be classified as overlapping distinct diseases, which could be caused by different causes, yet with similar morphologic, biologic and clinical manifestations.While traditional concepts of OA stress the primary and direct involvement of bone and cartilage in OA development however, it is now becoming more apparent that the synovium can also contribute to the pathophysiological underlying event of depletion of cartilage matrix.Conditions that respond to treatment with Arthramid(r)Vetinclude acute and chronic synovitis, osteoarthritis and degenerative joint disease. The cases that are suitable for treatment with Arthramid(r)Vetare those where lameness due to one of these conditions can be definitively identified and confined to the joint using an examination that includes clinical intra-articular analgesia, radiography, arthroscopy, MRI, CT and/or Scintigraphy.
It is crucial that an amnesty of information regarding ongoing infections or concomitant medications or surgery, or a fracture that could be a possibility is completed prior to injecting to ensure that there are no negative reactions or using the medication for conditions that are not those the ones for which it is approved.Case managementAfter the procedure, broad-spectrum antibiotics are used to reduce the chance of contracting an infection.Post injection instructions Ointments can be put directly on the injection site following treatment. A cold compress can be placed to the injection site to prevent of an swelling. A bandage is applied to the injection site, should it be it is. Local or systemic corticosteroids should not be administered to the animal within two weeks of injection of Arthramid(r)Vet,since this may mask a possible infection.
The animal should rest for 48 hours following the treatment.There are animals that experience discomfort within the first couple of hours following injection. There is a chance of haematoma and swelling around the area of the injection. After a period of 1-2 weeks, there is a chance that the animal will experience a temporary oedema or discomfort at the injection site because process of tissue integration begins. If the cause is not infections the reactions will be self-limiting and disappear within two weeks. Non-steroidal anti-inflammatory medicines (NSAIDs) can be used to ease pain and reduce swelling. Allergies to Arthramid(r)Vethave not been reported.As with any intra-articular procedure, an Arthramid(r)Vetinjection carries a risk of infection. A strict and safe injection techniques are essential. If there is an outbreak of infection, the use general spectrum antibiotics can be suggested as the first line treatment.
arthramid vet
The use of corticosteroids is not recommended in the event of an infection.The owner can be contacted for information.The animal’s owner must be aware of potential results, indications and contraindications, as well as precautions, warnings and the possibility of complications. The owner of the animal should be advised that in case of complications, the veterinarian who performed the Arthramid(r)Vetinjections should be contacted immediately fornecessary treatment.StorageArthramid(r)Vetmust be stored away by direct light. Don’t freeze. Do not keep syringes sealed for later use.
arthramid-vet ArthramidVetArthramid(r)Vetis a unique and patented hydrogel that offers an ingenuous alternative that is safe for vegetarians. OverviewThe new generation of joint treatment2.5% cross-linked polyacrylamide hydrogel82.5 percent success in resolving joint lameness that lasts for 24 monthsIntegrates into joint tissue, providing shock absorption for the joint. Biocompatible, safe, non-toxic and neuro-innocuous Share Enquire now Product description Arthramid(r)Vetis is used to treat non-infectious causes for joint pain in animals, which includes both the early and late phases of osteoarthritis (OA).Arthramid(r)Vetis is a non-absorptive, biocompatible, non pyrogenic neuro-innocuous, injectable polyacrylamide hydrogel for intra-articular injections in animals. Arthramid(r)Vetconsists of approximately 2.5% cross- linked polyacrylamide and 97.5% water forinjection. The hydrogel comes in a sterilized pre-filled 1 milliliter syringe that is sealed with an Luer locking fitting.
Product in detail Administration Arthramid(r)Vetis for intra-articular usage only and should be administered by a certified vet who is familiar with the procedure in the desired species. Animals can be sedated in order to reduce stress and discomfort. Aseptic guidelines must be strictly maintained at all times.A needle of 16-23G is inserted within the articular canal. Synovial fluid must be monitored. It is important to prevent damage to the intra-articular tissue as this could result in an extensive swelling that lasts for 24 up to 48 hours. Remove the protective tip cap from the Arthramid(r)Vetsyringe. Connect the syringe securely into the Luer lock socket on the needle. Check that the syringe is properly installed. If more than 1 mL of Arthramid(r)Vetis required forthe joint, detach the empty Arthramid(r)Vetsyringe from the hypodermic needle and repeat the process until the required dose is delivered.Dosages – EquineDue to Arthramid(r)Vet’sunique mode of action the following dosage recommendations have been made based on observed clinical responses to treatment;Distal
Interphalangeal (DIP/ Coffin)- 1-2 mLsMetacarpo/tarso-phalangeal (Fetlock)- 2 mLsShoulder- 3 mLsStifles- 1-2 mL per compartment or 3-4mLs formedial-femorotibial jointClinical trials conducted by the FDA have shown that horses that only partially respond to the initial treatment could be benefited by another dose 6 to 4 weeks after.Safety studies show that treatment for multiple joints on one animal can be secure.The mechanism of action is not all hydrogels made of polyacrylamide are identicalThe hydrogels made of polyacrylamide, though typically viewed as being equal, exhibit distinct differences in their composition, manufacturing quality, and injection methods in addition to their ability to work with tissues around them. These factors make up the safety and efficacy of every hydrogel.When injected into joints, Arthramid(r)Vet,a unique cross-linked 2.5 polyacrylamide hydrogel bonds in the synovial tissue by its capability for exchange of water molecules.
Over the course of 14 days, the gel will be incorporated in the synovial membrane as well as the surrounding tissue of the capsule’s interior through a combination of cell migration and vessel ingrowth, forming the thick, cushion-like lining comprised of a vessel-integrated gel that is covered by a brand newly hypercellular synovial cells lining.In the end, Arthramid(r)Vethas an enhancement effect on the synovium and joint capsule. It improves the elasticity and tensile strength in the capsule and improves its ability to carry the load. The theory is that the increase and cushioning leads to a decrease in mechanoreceptor activity within the capsule. This also leads to the development of a hypercellular and new synovial cell liner,
which improves the synovial fluid’s quality inside the joint. Both of these actions help to reduce synovitis as well as its subsequent consequences. Unlike other hydrogels Arthramid(r)Vetbecomes fully integrated into the surrounding tissue (rather than encapsulated) and is therefore long lasting. Arthramid(r)Vetdoes not trigger any foreign body reaction or cause infections. Arthramid(r)Vetis is non-pyrogenic, neuro-innocuous and does not contain heavy metals. Case selection Understanding the complexities of disease processes related to joint pain is an ongoing challenge in the clinical setting and, as in any other disease process the ability to accurately diagnose is vital. Arthritis is a term used to describe joint inflammation and can develop after one or repeated episodes of trauma. The term encompasses synovitis capsulitis, as well as osteoarthritis (OA).
These diseases are a collection of distinct diseases, which could be caused by different etiologies but with similar morphologic, biologic and clinical manifestations.While traditional concepts of OA focus on the central and primary role of bone and cartilage in OA development It is becoming increasingly apparent that synovium is also a contributor to the main pathophysiological cause of depletion of cartilage matrix.Conditions that respond to treatment with Arthramid(r)Vetinclude acute and chronic synovitis, osteoarthritis and degenerative joint disease. Patients who are suitable for treatment with Arthramid(r)Vetare those where lameness resulting from one of these conditions is identified and confined to the joint through an examination that includes clinical intra-articular analgesia, radiography, arthroscopy, MRI, CT and/or Scintigraphy.
It is imperative that an analysis of any evidence of an ongoing infection or concomitant medications or surgery, or even a possible fracture is completed prior to injecting to avoid possible negative reactions or using the drug for conditions other than those that for which it is recommended. Case management After the procedure, broad-spectrum antibiotics can be used to reduce the risk of getting sick. Post injection instructions A ointment may be applied to the injection site right away following treatment. A cold pack may be applied to the injection site in the event an swelling. A bandage may be placed to the injection site, should it be it is. Local or systemic corticosteroids should not be administered to the animal within two weeks of injection of Arthramid(r)Vet,
since this may mask a possible infection. The animal should rest for 48 hours following the treatment.There are animals that experience discomfort within the first couple of hours following injection. Additionally, there is a chance of haematoma or mild oedema around the site of injection. After a period of 1-2 weeks, there is a small chance that the animal could suffer from a brief oedema and tenderness around the site of treatment because tissue is integrating. If it isn’t caused by an inflammation These reactions are self-limiting and disappear within two weeks. Non-steroidal anti-inflammatory medications (NSAIDs) are used to ease pain and reduce swelling. Reactions with allergies to Arthramid(r)Vethave not been seen.As with any intra-articular procedure, an Arthramid(r)Vetinjection carries a risk of infection. A strict and safe injection techniques are essential. If there is an outbreak of infection using general spectrum antibiotics can be suggested as the first line treatment.
arthramid for horses
The use of corticosteroids is not recommended in the event of an infection.Contact the ownersThe animal’s owner must be aware of potential results, indications and contraindications, as well as precautions, warnings, and possible complications. The owner of the animal should be advised that in case of complications, the veterinarian who performed the Arthramid(r)Vetinjections should be contacted immediately fornecessary treatment.StorageArthramid(r)Vetmust be stored away against direct sunlight. Don’t freeze. Don’t store syringes that are sealed to use later.Information ForUse (IFUTestimonialI was one of the first vetsto use Arthramid(r)Vetworldwide and have now used Arthramid(r)Vetforover 10 years in our clinic. We have injected more than thousand horses and discovered that it is extremely secure. It’s been a huge revolution in the way we manage OA among our patients.Dr Marc Koene DVM – Founder and Partner at Tierklinik Lusche GmbH EquineHospital in Northern Germany
Polyacrylamide Gel (Arhtramid-Vet/Noltrex-Vet)Polyacrylamide products are the ideal solution to cases of osteoarthritis that are advanced in horses.These products are injected directly into the joint to provide visco-supplementation (lubrication/cushioning) and re-establish healthy joint lubrication using hydrogel. The Noltrex-Vet and Arthramid-Vet functions method to hyaluronic acids (HA) but they don’t break down as fast over time. This means they are able to be a significant therapy over the long term and help for joints that are in poor condition.
We have been using Arthramid-Vetand Noltrex-Vetforseveral years with extremely impressive results even in otherwise refractory or severe cases. Polyacrylamide gel can provide long term results even in certain cases of advanced osteoarthritis in horses. This gel is injected directly into the joint to provide visco-supplementation (lubrication/cushioning) and re-establish healthy joint lubrication using hydrogel. Arthramid-VetandNoltrex-Vetfunction in manner to hyaluronic acid (HA) but they don’t degrade at the same rate over time and don’t require metabolization for their functional. They are able to immediately provide substantial long-term therapy and help for joints with compromised. Our practice has been using polyacrylamide gel for several years with remarkable results, even in severely refractory instances.
4
The information typically consists of the following information: ArthramidVetIndicationsAvertissements and warnings for Arthramid VetDosage and directions for Arthramid VetArthramid Vet Instruction For Use Arthramid Vet Caution The law of the United States restricts the sale of this device to or upon the instruction of a licensed physician and it is only utilized by a registered vet for the use of intra-articular administration.
Product Description Arthramid(r)Vetis is a non-resorbable, injectable transparent, hydrophilic, transparent gel that is intended for intra-articular administration in horses only.Arthramid(r)Vetconsists of a backbone of cross-linked polyacrylamide, with water molecules loosely bound to the polymer matrix. The nominal percentages of Arthramid(r)Vetgel are 2.5 percent cross-linked polyacrylamide, and 97.5 percent non-pyrogenic water for injection. Arthramid(r)Vetis biocompatible and non-biodegradable, and is sterilized using moist heat.Arthramid(r)Vetis comes in pre-filled, sterile 1mL syringe that is sealed with an Luer Lock fitting.
It is designed to be injected using the sterile 16-23G hypodermic needle.IndicationArthramid(r)Vetis used to treat non-infectious joint disease in horses, which includes both the later and early stages of osteoarthritis, as well as degenerative joint diseases.ContraindicationsArthramid(r)Vetis is not recommended for injection into joints with active infection as well as infected joint soft tissues, or skin that is infected that covers the joint.Warnings* Do not inject Arthramid(r)Vetintravascularly.
Injections into blood vessels could result in a vascular obstruction and lead to embolism.* Don’t make use of Arthramid(r)Vetif the package has been damaged or opened.* Do not re-sterilize Arthramid(r)Vet.Don’t mix Arthramid(r)Vetwith another product.* Do not apply Arthramid(r)Vetin together with other product intended for intra-articular injection for a period that is not less than 30 days.* Don’t make use of Arthramid(r)Vetafter the expiration date which is printed on the package.PrecautionsArthramid(r)Vetis is not recommended for use in horses with chronic or acute diseases who are that are treated with systemic corticosteroids or antibiotics.
The injection site must be cleaned before treatment.Medical history records addressing ongoing infections, concomitant medication, surgery, etc. must be reviewed prior to injecting to ensure that there are no infections.When used, syringes as well as needles must be handled with care because they could pose a biohazard. Be sure to dispose of them according to the accepted medical practices and local regulations, federal and state laws.
Method of AdministrationArthramid(r)Vetmust have the treatment administered by a veterinarian who is who is trained in these kinds of procedures.Arthramid(r)Vetis delivered via percutaneous infiltration into the articular space of the joint affected, delivering the amount required depending on the joint to be infected.Dose RecommendationThe following dosage guidelines were formulated in light of the clinical reactions observed to the administration of medication;* Distal Interphalangeal (DIP/ Coffin) – 1-2 mL* Metacarpo/tarso-phalangeal (Fetlock) – 2 mL* Tarso-metatarsal (TMT)/ Distal-intertarsal (DIT) – 1 mL* Tarsocrural – 2-3 mL* Shoulder – 3 mL* Stifles – 1-2 mL per compartment or 3-4 mL formedial-femorotibial jointOne dose of the amounts listed above is sufficient to get the desired effect.
The results of clinical trials published in the literature suggest that horses who are not fully responsive to initial treatment could receive a second dose 4 to six weeks after the initial treatment. Studies have shown that simultaneous treatments of several joints within an animal in the same is secure. Peri-Operative Procedures1. Relax or calm the horse.2. Cut the hair that covers the joint that will be injection. To prevent the spread of infection washing and disinfecting thoroughly within a radius of 3 inches around the injection location. The injection of Arthramid(r)Vetmay be administered using local anaesthesia.Aseptic technique must be used to avoid contamination of the sterile Arthramid(r)Vetsyringe and injection site.
The hypodermic needle 16-23G should be inserted into the articular space. Synovial fluid must be monitored. Be careful to avoid causing unnecessary damage to the intra-articular tissues, since this could result in the swelling spreading over 24 or 48 hours. Remove the protective tip cap from the Arthramid(r)Vetsyringe. The syringe is then tightly inserted into the lock socket of the needle hypodermic. Check that the syringe is properly installed prior to using.
If more than 1 mL of Arthramid(r)Vetis required forthe joint, detach the empty Arthramid(r)Vetsyringe from the hypodermic needle and repeat the process until the required dose is delivered. When the procedure is complete, take out the hypodermic needle as well as the syringe. Dispose of them according to accepted medical practice and local as well as federal and state regulations. Cover the injection area with a sterile, non-adhesive dressing.
Post-Operative Procedures Ointment can be sprayed directly on the injection site following treatment. A cold compress can be place to the injection site to prevent an swelling. A bandage is recommend on the injection site, if there is a possibility.
Non-steroidal anti-inflammatory medications (NSAIDs) can be used to ease pain and reduce swelling. Allergies to Arthramid(r)Vethave never been seen.ComplicationsAs with any intra-articular procedure, an Arthramid(r)Vetinjection carries a risk of infection. Regular precautions and a strict aseptic injection procedure are vital. If there is an outbreak of infection it is recommend to use broad-spectrum antibiotics is suggest as a first-line treatment. The use of corticosteroids is not recommended in the event of an infection. Certain animals may experience pain in the first few hours after administration. Additionally, there is a chance of edema or hematoma on the injection site.



Reviews
There are no reviews yet.